Preparing the Certification of Software as a Medical Device: A European Regulatory Analysis and Case Study on the Clynx® Platform
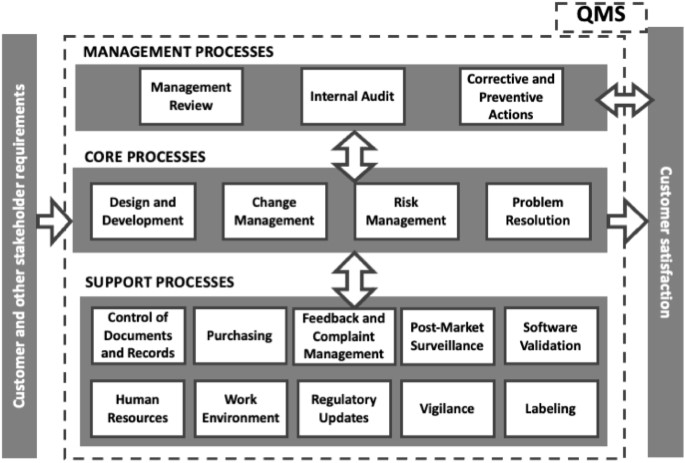
This study explores the certification pathway for Class IIa medical device software, in accordance with the Medical Device Regulation 2017/745 (MDR). The methodology focused on the process for obtaining a Conformité Européenne (CE) marking, emphasizing the compilation of Technical Documentation, the nuances of Clinical Evaluation, and the establishment of a Quality Management System. An application to an industry case study is also described. The certification process is conducted in collaboration with Notified Bodies, ensuring adherence to the stringent requirements set forth by the existing regulatory framework. See more.